Research Study 1

Fig. 1. Flabellina trilineata

Fig. 2. Pathway of nematocysts through an aeolid nudibranch's gut system from mouth to cnidosac

Fig. 3. Arrangement of nematocysts (black dots representing at least two types) within a nudibranch's cnidosac
Many species of aeolid nudibranchs, including Flabellina trilineata (Fig. 1), sequester undischarged nematocysts, athought to be used in their own defense. The nematocysts are housed in special sacs or cnidosacs located in the tips of the cerata. Each cnidosac is lined with a single-layered epithelium composed mainly of phagocytosing cells called cnidophages. Nematocysts that are eaten are sorted in the stomach and shunted undischarged into the diverticula of the digestive gland that extend into the cerata. From there they pass through a narrow canal that opens into each cnidosac (Fig. 2). On entry into the lumen of the cnidosac the nematocysts are phagocytosed by the cnidophages. Within the cnidophages the nematocysts may lie parallel with one another, or be arranged in circles or bouquets depending upon the species, with their discharge ends mostly pointing outwards (Fig. 3). When Flabellina and other nematocyst-bearing aeolids are disturbed or irritated, as by a potential predator, they curl their bodies and bristle their cerata. Special muscles surrounding the cnidosacs squeeze the nematocysts from their sacs and enclosing cnidophage cells, and force them out a cnidopore at the tip of the ceras. Once they contact seawater they discharge. In theory, some of the nematocysts penetrate the predator and it withdraws. Presumably, those nematocysts that penetrate are ones that are “backed up” against the cnidosac or the discharged cell mass; otherwise, a free-floating capsule would likely discharge its thread ineffectively.
NOTE bristling makes it difficult for a potential predator to avoid touching the cnidopore ends of the cerata. Studies on cnidosac discharge in an Atlantic nudibranch that specialises in eating the polyp stages of jellyfishes show that release of nematocysts from a cnidosac is in bunches, thus allowing for second, and perhaps more, discharges. The scientists assess the potential defensive efficacy of these discharges by touching the cerata to the soft parts of their own lips (WARNING: exercise caution when thinking about using your own tongue and lips to assess the stinging potential of nematocysts, especially those from hydroids. Even west-coast anemone species that can be touched by the finger with impunity can produce long-lasting and potentially serious allergic responses if touched to more sensitive skin areas)
NOTE action and reaction being equal
Kalker & Schmekel 1976 Zoomorph 86: 41
Research Study 2

Fig. 1. Glaucus atlanticus showing predominant cerata with nematocysts in their tips
Unfortunately, the above "defensive" scenario has never been demonstrated in controlled experiments. There are anecdotal reports of fishes shaking their heads after biting at the cerata of an intended prey aeolid nudibranch, but most or all such reports lack scientific rigour. There is no question that the cnidosac-borne nematocysts can, in fact, sting (see the "NOTE" in Research Study 1 above). Furthermore, swimmers in Australia are reported being stung by contact with swimming nudibranchs Glaucus (Fig. 1). This predator favours Portuguese-man-of-war Physalia spp. and related siphonophores as food, and is known to sequester highly toxic nematocysts from the prey in its cnidosacs. The Glaucus observation is certainly convincing evidence for a defensive role for the nematocysts but, of course, how it functions in nature is not known.
Thompson & Bennett 1969 Science 166: 1532
Research Study 3

Fig. 1. Flabellina verrucosa
One of the earliest studies on nematocyst cycling and selection of specific nematocysts to sequester involves Flabellina verrucosa eating hydroids in New Hampshire. This species is cosmopolitan in distribution and also inhabits the west coast of north America, so the study may stimulate local research interest. The authors proceed in their investigation by collecting Flabellina and examining what kinds of nematocysts they have in their cnidosacs. The examination shows that the dominant nematocyst at the time of collection is a kind known as a microbasic eurytele. However, when given three different hydroids to eat in separate experiments, the researchers find that this dominant type is replaced as follows:
- when eating the hydroid Hydractinia echinata with four types of nematocysts, the dominant one in the cnidosacs after several days is a type known as a microbasic mastigophore
- when eating the hydroid Tubularia crocea with four types of nematocysts, the dominant one in the cnidosacs after several days is a type known as a stenotele (a toxic “killing” kind of nematocyst)
- when eating the hydroid Obelia geniculata that has a single type of nematocyst known as a basitrichous isorhiza, after several days this becomes the dominant one in the cnidosacs
Thus, in all three treatment groups the originally dominant nematocyst type, the microbasic eurytele, is replaced by a different type. The authors note that several hydroid species are present in the collection area that could have been eaten by the nudibranchs so, based on what they have found so far, the nudibranchs must have been specialising on one or more species that possess this type of nematocyst. The authors conclude by commenting that nematocysts may be selected on bases of toxicity or how readily they lend themselves to the mechanics of transport through the gut (this "motherhood statement" is not supported by any evidence; however, it is provocative enough to justify further investigation).
NOTE the authors also note that Aeolidia papillosa eating the plumose anemone Metridium senile selectively sequesters two types of nematocysts from 6 types available
NOTE the authors of the present study and Research Study 9 to follow have different ideas of the nematocyst complement in this hydroid species. Someone should look into this in relation to habitat effects on nematocyst complement of various hydroid species...perhaps they are not as constant as one might think
Day & Harris 1978 Veliger 21: 104
Research Study 4

Fig. 1. Structure of cnidosac within a ceras
Within the cells of the cnidosac the nematocysts are contained within vacuoles. Many nematocysts are contained in each cell (Fig. 1). On stimulation of a ceras, special muscles at the base of the cnidosac contract. This ruptures the integrity of the cnidosac, and squeezes cells and nematocysts out at the top, either through a permanent hole, the cnidopore, or through a thin membrane covering the pore, depending upon species. On contact with the outside environment the nematocysts discharge, but only if the cell containing them is itself ruptured.
NOTE in a typical aeolid nudibranch there are about 3,000 nematocysts within each cnidosac. So, with a total of 100 cerata, there would be some 300,000 functional nematocysts for the nudibranch to use in its own defense
Greenwood & Mariscal 1984 Tissue & Cell 16: 719
Greenwood & Mariscal 1984 Mar Biol 80: 35
Research Study 5

Fig. 1. Aeolidea papillosa
A neat technique for sampling nematocysts released from aeolid cnidosacs is described for Aeolidia papillosa (Fig. 1). A glass microscope slide is coated in saliva, dried to tackiness, then gently pressed on the cerata, which have been caused to bristle by poking the animal. The cnidosacs release their contents and the nematocysts discharge onto the saliva, allowing close examination of them at a later time, perhaps after staining or other treatment. The author suggests that the mechanics of how the nematocysts discharge when this method is used supports the hypothesis that the primary function of the sequestered nematocysts is for defense.
NOTE apparently stopcock grease works equally well
Gaulin 1982 Veliger 25: 171
Research Study 6
How does the sequestration of undischarged nematocysts work? How can a nematocyst pass by the jaws and radula without discharging? One often reads explanations that mucus secreted by the nudibranch inhibits discharge of the nematocysts. However, as nematocysts take a few days to develop, another idea, quite original and not tested, is that the functionally mature ones discharge when the snail eats them, while the functionally immature ones are moved into the digestive-gland diverticula and then to the cnidosacs. Development of the nematocysts is then completed within the cnidosacs. Feces of aeolids are usually filled with discharged nematocysts. The means by which a host would differentiate between discharged and undischarged capsules in the stomach or digestive gland is not known.
NOTE one would think that this could be tested simply by studying the nematocyst contents of the digestive-tract extensions into the cerata to check for degree of maturity. This is actually done by the researchers, described in their Research Study to follow
NOTE the gut lining of nematocyst-eating nudibranchs typically is well vacuolated, that is, containing balloon-like inclusions. Another possibility, then, is that these vacuolations provide insulation against the stings
Greenwood & Mariscal 1984 Mar Biol 80: 35
Research Study 7

Fig. 1. Percentages of immature nematocysts in cnidosacs of Atlantic-coast
Spurilla neopolitana superimposed on a drawing of a cnidosac in the west-coast nudibranch
Flabellina trilineata (proportions in the latter species are actually not known)
Courtesy Kälker & Schmekel (1976)
If the above description is true, then immature nematocysts should be found within newly developing cnidosacs, and this is indeed the case. Studies in Florida on Spurilla neopolitana1 in which cerata are experimentally removed from recently fed individuals reveal an initially higher percentage2 of immature nematocysts in the lower portions of the cnidosacs than in the upper portions (Fig. 1). This suggests a movement and a maturation within the cnidosacs from the digestive gland at the lower end to the cnidopore at the upper end. Nematocysts typically mature in 2 - 4d and have a finite life span. Presumably, the old non-functioning nematocysts3 are cast out or absorbed by the host. If Spurilla is starved for 3d, the number of immature nematocysts in their cnidosacs drops from about 150 to 30, suggestive of an interrupted “supply line”.
NOTE1 an anemone-eating aeolid with some features in common with the west-coast Aeolidiella chromosoma. A typical cnidosac in Spurilla contains over 3,000 nematocysts. The study is included here with the hope that someone will do a similar study on a west-coast aeolid
NOTE2 a point not discussed by the authors is that the proportions listed here don’t seem high enough to account for a steady movement of nematocysts through the cnidosac via a casting out or absorption as they senesce. For example, if a nematocyst matures in 2 - 4d and has a life span in the cnidosac of, say, 10d, then wouldn’t proportions from base to tip be more likely to be in the ranges of 40, 10, and 1%...just as a guess?
NOTE3 these ideas contrast an earlier theory that non-functioning and/or senescent nematocysts are disposed of by the host voluntarily casting off its cerata. Ceratal autotomy does indeed happen, but whether this is its function is not known. It would seem a wasteful strategy, especially in view of the cycling option proposed here
Greenwood & Mariscal 1984 Mar Biol 80: 35
Kälker & Schmekel 1976 Zoomorph 86: 41
Research Study 8
Fig. 1. While sunflower stars Pycnopodia helianthoides are known to rear back defensively from contact with many different types of cnidarians, such a response to the tiny nudibranch Hermissenda crassicornis has not yet been observed (the photograph you see here has, of course, been "staged")

Fig. 2. Crossaster papposus

Fig. 3. Hermissenda crassicornis with damaged, missing, and perhaps regenerating cerata, cause not known
In tests at Bamfield Marine Sciences Centre, British Columbia of a possible defensive roles of nematocysts in aeolid nudibranchs, the cerata of Hermissenda crassicornis are placed in contact with sea stars Pycnopodia helianthoides (Fig. 1) and Crossaster papposus (Fig. 2). Although discharged nematocysts could be seen in the tube feet of Crossaster, the authors conclude that the nematocyst do not play a major defensive role against these and other potential predators (also tested are hermit crabs and sculpins). Rather, they suggest that the cnidosacs may function primarily as a storage device for safely sequestering nematocysts that could damage the digestive system, with ceratal autotomy being used to rid the body of these unwanted nematocysts. This reiteration of an old idea for the function of the cnidosacs underscores the need for further research. The authors note a prevalence of what appears to be predator-induced damage to cerata in one of their study populations of Hermissenda, so identification of the modus operandi of various “cerata-attacking” species in the laboratory could allow identification of predatory attacks in the field
Miller & Byrne 2000 Invert Biol 119: 167
Research Study 9

Fig. 1. For each nematocyst type the undischarged state is shown to the Right of the discharged state
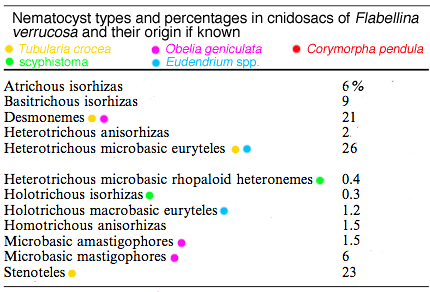
The most common nematocysts that Flabellina verrucosa sequesters are heterotrichous microbasic euryteles from the hydroids Tubularia crocea and Eudendrium spp. Next most common are stenoteles from Tubularia crocea, followed by desmonemes from Tubularia crocea and Obelia geniculata
Other studies on nematocyst complement in Flabellina verrucosa in the Gulf of Maine show that while it is a generalist predator of hydroids, it also eats jellyfish scyphistomae. About 70% of Flabellina’s nematocysts come from the hydroids Tubularia crocea and Eudendrium spp. Most of these nematocysts are of a type known as heterotrichous microbasic euryteles, which are predominant in these species (Fig. 1). Although only about 0.5% of Flabellina’s nematocysts come from scyphistomae (see Table: green dots), these polyps are selected over any others in choice tests, perhaps because of the toxicity of their nematocysts. Considerable variability exists in nematocyst complement in F. verrucosa from different geographical areas and, when switched to different diets, Flabellina’s nematocyst complement changes to match that of the new diet within 2wk . For example, within 2wk on a diet of scyphistomae, over 96% of the nematocyst complement is made up the two nematocyst types common in that prey (holotrichous isorhizas and heterotrichous microbasic rhopaloid heteronemes). On a diet of tunicates, which contains no nematocysts, the cnidosacs of Flabellina still retain some hydroid-derived nematocysts after 2wk. Although supporting evidence is not provided, the author suggests that the quick turnover of nematocysts with change in diet in Flabellina may give it the potential to “arm” itself quickly against specific predators. A good idea, indeed.
NOTE tunicates used are Botrylloides violacaeus and Didemnum sp.
NOTE is the author implying by this that on encounter with a specific predator Flabellina seeks out certain prey from which it derives the specific nematocyst weaponry to discourage that predator? If true, this is a good strategy, indeed!
Frick 2005 Mar Biol 147: 1313
Test Your Understanding
So, what have we learned from these studies? What features of a nematocyst are important in its selection by a nudibranch, specifically, by Flabellina verrucosa? Consider these answers, then seek out explanations.
[Click each option to see commentary]
-
Availability.
Option a:
Yes. If turnover time is short for a particular nematocyst in the cnidosac, then it would be advantageous if that type were plentiful in the prey.
[hide]
-
Transport suitability.
Option b:
Yes. Fast-maturing nematocysts, for example, might be unsuitable simply because fewer of them would be available for transport to the cnidosacs.
[hide]
-
Toxicity.
Option c:
Possibly. While highly potent nematocysts would seem to be desirable, ultimate selection of a particular nematocyst type might depend on a balance between availability, transport suitability, and toxicity.
[hide]
-
Predator type.
Option d:
Yes. There is evidence that a nudibranch may select nematocysts based on a “risk-assessment” of the presence its own predators in its habitat (see Research Study that follows this quiz). For example, a toxic penetrant type might be useful around predatory fishes or sea stars, or a tangling type where small predatory crustaceans are common.
[hide]
Research Study 10

Fig. 1. Effect of upstream presence of predators on selection of a certain highly toxic nematocyst by Flabellina verrucosa
The aeolid nudibranch Flabellina verrucosa primarily eats hydroids and jellyfish scyphistomae, from which it extracts nematocysts for incorporation into its cnidosacs. If fed on hydroids Tubularia spp. and Obelia geniculata the nematocysts1 sequestered reflect what is available in the prey, as has been seen in other studies. Even though the predators of Flabellina are not known, a question asked by a researcher in Maine2 is whether the presence of a potential predator might influence the type of nematocyst that Flabellina sequesters. The answer is ‘yes’, as the upstream presence of sea stars Crossaster papposus and cunner fishes Tautogolabrus adspersus (an Atlantic species) over a 2wk period in laboratory experiments causes significantly increased uptake of highly toxic microbasic mastigophore3 nematocysts over control animals (Fig. 1). In contrast, upstream presence of green crabs Carcinus maenas does not influence uptake. An ability to modify the nematocyst complement in its cnidosac would theoretically enable a nudibranch to adapt its defensive weaponry to combat predators specific to an area in which it lives. Are the sea stars and fishes tested really natural predators of Flabellina? This we may never know, but the research question addressed is unique and the results certainly point to a need for further work on other species, especially west-coast ones. An interesting aspect of this research idea is that in the absence of knowledge of natural predators of nudibranchs, why not let the nudibranch itself tell us by its nematocyst complement which are its predators?
NOTE1 according to the author the nematocyst complements in the two genera of hydroids are mutually exclusive, and thus present a range of nematocysts (at least 9 different types) from which Flabellina can choose
NOTE2 Flabellina verrucosa has a circumboreal distribution, and is found in the north Pacific and north Atlantic regions. This in part explains the inclusion of several east-coast studies in this section of the ODYSSEY; however, the main reason is to encourage west-coast scientists to get busy researching this fascinating topic
NOTE3 the author provides uptake data for all 9 nematocyst types. Of these, only microbasic mastigophores differ significantly between control and experimental treatments, and then only for two of the three potential predators tested. Two other nematocyst types show slight but significant reductions in the treatment group; all other pairings are not significantly different
Frick 2003 Biol Bull 205: 367
Research Study 11

Fig. 1.
Flabellina iodinea showing brightly coloured (to our eyes) cerata with tips containing nematocyst-filled sacs. Little is actually known about the how the sacs function in defense in these or other aeolid nudibranchs
Courtesy Gary McDonald, Long Marine Laboratory, Santa Cruz, California and CalPhotos

Fig. 2. In these actual photos by the authors, note the conspicuous size difference between predator and prey. The Left photo-pair shows aversive behaviour to Flabellina iodinea. The Right photo-pair shows the same predator 20min later attacking and eating another nematocyst-defended aeolid, Hermissenda crassicornis. Not all naive predators show the same aversive responses to Flabellina...about half of the 40-or so Pleurobranchaea tested in the study simply attack and eat up the prey
Learned-avoidance behaviour in predators of nematocyst-bearing nudibranchs has not been well studied, mainly owing to lack of knowledge of which predators might be involved. One exception to this last is the large notaspidean (side-gilled) opisthobranch Pleurobranchaea californica, which is known to attack and eat a number of different nudibranchs. Research at Hopkins Marine Station, California shows that contact of the big predator with the much smaller nematocyst-bearing aeolid Flabellina iodinea (Fig. 1) can elicit rapid aversive responses in the former (Fig. 2), a behaviour quickly learned and remembered (experienced Pleurobranchaea show strong avoidance behaviour for several days after exposure). The aposematic learning response is not visual, for the predator lacks the sensory capability for this; rather, it is a response to chemical odour of the prey. In support of this the authors note that some attacks stop prior to contact, suggesting response of the predator to water-borne stimuli, and this is confirmed in other experiments involving extracts of Flabellina. These observations add to a growing body of knowledge of associative learning, memory, and aposematic odour-aversions in opisthobranch molluscs. One wonders, however, whether another research-protocol step, possibly a definitive one, might not be taken, and that is to create a "nematocyst-free" aeolid prey by feeding it for a time on non-cnidarian prey such as tunicates or mussels. This can be done with some species, and perhaps also with F. iodinea.
NOTE termed aposematic odour-learning . Much of the previous research on aposemetism, that is, warning to potential predators that attacking a certain prey might lead to unpleasant consequences, has been colour-based, as seen in the "advertisement" colours of toxic bees, wasps, ants, and butterflies, but odour-based (chemical) warnings are known from a few species to work in a similar way
Noboa & Gillette 2013 J Exp Biol 216: 3231
Research Study 12

Fig. 1. Length-wise section of a ceras of Aeolidia papillosa. Features of note are the closed-over cnidopore, the long microvilli of gastrodermal cells leading into the cnidopore region, and the muscle sheaths, each comprising juxtaposed circular and longitudinal muscle layers. Note the apparent lack of cilia on the gastrodermal cells extending from the ceratal extension of the digestive gland into the cnidosac. Lack of cilia prompts the question as to how the nematocysts are moved along in the digestive gland and, once into the cnidosac, how they are sorted and organised within the cnidophage cells.

Fig. 2. Close view of the ruptured cnidopore of a ceras of Aeolidia papillosa with several nematocysts in the process of being squeezed out preparatory to discharging.
Researchers at the White Sea Biological Station, Arkangel, on the north coast of Russia describe the mechanism of operation of the cnidosacs of Aeolidia papillosa. Chief features of an undischarged cnidosac structure shown in Fig. 1 are nematocysts (termed kleptocnidae by the authors) enclosed in so-called cnidophage cells, and two layers of muscle cells, one surrounding the cnidosac and one around the ceras itself. Note the cnidopore of the intact cnidosac is covered by ciliated epidermis. The nematocysts (termed kleptocnidae by the authors) within the cnidosac reside in vacuoles within cnidophage cells. On stimulation, such as by a predator, the muscle layers around the cnidosac and ceras contract with enough force to rupture the epithelium covering the ceras tip and expel the nematocysts. The nematocysts appear to be guided to the cnidopore by the thick mass of microvilli that orients and funnels them out (shown in blue in Fig. 1). The authors note thatt despite the emergence of discharging nematocysts from the cnidopore (Fig. 2), the integrity of the epithelium layer seems little disrupted. They suggest that this needs further study. The potentially damaging effect of newly released discharging nematocysts on the animal's own cerata may be ameliorated by the layer of chitinous spindles in the outer epithelium (Fig. 1).
NOTE klepto+cnidae: "stolen nettles" G. a not-well-used bit of jargon that however is self-explanatory
NOTE the microvilli are apparently a unique feature of this aeolid species; they may function to orient correctly the nematocysts on expulsion
Vorobyeva et al. 2017 Doklady Biol Sci 476: 196
Research Study 13
Given that an aeolid nudibranch such as Hermissenda crassicornis may eat a variety of hydroid prey, each with its own specific complement of nematocysts, the questions arise as to whether the nematocysts are uniformly distributed among the cerata), and the duration of sequestration of each. This is investigated by a researcher at Bamfield Marine Science Centre who collects seven Hermissenda nudibranchs and itemises the nematocyst complement in four cerata from each individual, one ceras from each of four quadrants on the dorsal surface (Fig. 1 shows the 11 types of nematocysts found). Results show that the nematocysts sequestered do indeed differ between different cerata, and the author cautions other researchers to be mindful of this when considering their own studies. Sixteen additional Hermissenda are investigated for nematocyst duration in the cerata. Half are fed on crushed mussel flesh, and half are fed a mixture of mussels and hydroids Obelia sp. Cerata are checked for nematocyst complement every 3 - 5d over a 42d period. By 42d all 8 mussel-fed individuals have significantly reduced nematocyst numbers as compared with ones fed a mixture of mussels and hydroids.
NOTE although not the original intent of the study, would its value not have been increased by comparing ceratal nematocyst complements at the start with nematocysts available in prey in the immediate habitat? Also, the author does not mention nematocyst types in Obelia at the start of the 42d experiment. There might have been good information available on preferential sequestration of nematocysts from this prey species, and how this might change over time, had the author chosen to present it
NOTE 42d is obviously an arbitrary choice, and represents a minimum estimate of retention time. For a true estimate one would need to know how long various nematocysts were resident in the cerata prior to when the experiment started

Fig. 1. Of 11 different nematocyst types found in cerata of Hermissenda crassicornis, Nos. 1 - 4 are the most common. The author does not name the different types even though this information is readily obtainable
Courtesy The author and Can J Zool
Anthony 2020 Can J Zool 98: 808
Research Study 14

Fig. 1. Nudibranch Berghia stephanieae

Fig. 2. Early feeding stage of Berghia stephanieae just a few days old. Brown matter in the digestive gland is primarily brown-coloured dinoflagellates that are symbiotic with the anemone. The inset view shows nematocysts being shunted towards the early developing cnidosac

Fig. 6. Five-second track of a single nematocyst being moved from the digestive gland (on the into the Right-hand side) into the cnidosac and then back out again. Clearly, some sort of fast-acting selection process is operating, thought by the authors to be the phagocytic cells themselves making the choices

Fig. 7. Cartoon showing nematocysts entering the cnidosac with one in the process of being phagocytised into one of the lining cells (see inset). Dinflagellates are also seen to enter the cnidosac, but are quickly shunted back into the digestive gland
Information on sequestration of nematocysts during early development of the aeolid Berghia stephanieae (Fig. 1) is provided by researchers at Scripps Institution of Oceanography, La Jolla, California. From an early age Berghia's major prey are anemones Exaiptasia diaphana, and even before the cerata appear in development nematocysts are being removed from food matter in the digestive-gland into the newly forming cnidosac (Fig. 2). Three types of nematocysts are selectively removed including the microbasic p-mastgophores, which is the largest type found in the anemone. At some stage in the process the nematocyst are enclosed in membranes and taken up or phagocytosed into the large gastrodermal cells lining the cnidosac (Figs. 3 -5). The authors note that some nematocysts my be moved into the cnidosac, then a few seconds later be moved out (Fig. 6). The authors propose that the phagocytic cells are responsible for the sorting, perhaps relying on split-second chemical assessments (..no good? spit it out...!). Dinoflagellates can also be seen entering the cnidosac, but are also squeezed back out within a second or two as none are useful within the cnidosacs (Fig. 7). As you can see, the figures provided by the authors are extraordinary, the entire account is informative, and the authors are to be congratulated on a fine job.

Figs. 3 - 5. Three onfocal images of early developmental stages of Berghia stephanieae showing cerata and cnidosacs growing and progressively being filled with undischarged nematocysts. Inset views in each case show close-ups of the developing cnidosacs

Fig. 4. Purple-stained matter in these figures is digestive-gland contents, primarily dinoglagellates that were symbiotic in the prey anemones

Fig. 5.
Goodheart et al. 2022 Front in Zool 19: 16




































 Nudibranchs & relatives
Nudibranchs & relatives