Most of the 37 or so world species of sea hares Aplysia release a purplish-coloured ink when disturbed. Additionally, some species release a white “opaline” secretion, also thought to be used in defense. Species eating red seaweeds also have a variety of secondary metabolites in their skins and digestive glands that may be defensive. This section starts with a review of defenses in sea hares, and should be a “must-read” for anyone interested in the subject. After this entry the Research Studies are presented in chronological order.
Research Study 1

Fig. 1. Drawing of sea-hare Aplysia features relating to ink and opaline secretion
An article from researchers at the University of Washington and Friday Harbor Laboratories, Washington provides clarification of several generalisations and misconceptions relating to ink and ink release in sea hares Aplysia spp. (Fig. 1), and a brief review of these may aid in understanding the results of Research Studies presented later. Points of clarification about the ink include:
- all sea hares have ink glands, most releasing a purple secretion, but some releasing white ink
- the ink gland is located in the mantle directly over the gill (ctenidium), with the ink being released through pores on the ventral surface of the gland
- the ink is composed primarily of pigments (phycoerythins) derived from red-algal foods, but does not contain secondary metabolites of the algae
- possible functions of the ink include anti-predator and predator-warning
- release of ink is not necessarily high threshold or all-or-none, as has been described by other researchers.
Points of clarification about opaline secretion: 1) all sea hares have opaline glands, located beneath the floor of the mantle cavity, 2) opaline secretion is released less readily and in smaller quantities than ink, 3) the secretion is highly proteinaceous and viscous, 4) its production is not dependent upon red algae being eaten, and 5) its function is not known. The authors comment that this part of sea-hare biology needs to be researched.
NOTE chemicals in the body that seem to have no role in metabolism; rather, thought to have defensive functions
Johnson & Willows 1999 Mar Freshw Behav Physiol 32: 147
Research Study 2
Of the two west-coast aplysiid species, only Aplysia californica releases a purple ink that is derived from a diet of red seaweeds; the sympatric A. vaccaria does not. Early experiments at the Scripps Institution of Oceanography, California on feeding of A. californica on various red algal and biliprotein diets show that the main component of the ink, termed aplysioviolin, is derived from phycoerythrin of red algae, such as Laurencia pacifica. On a diet of brown algae Egregia laevigata, A. californica becomes facultatively de-inked. When returned to a diet of red algae the ability to produce ink is restored. Within 72h some of the consumed phycoerythrin is transferred to the egg mass, where it imparts a mauve-purplish colour. The authors do not consider the aplysioviolin component of the ink to be defensive; rather, they suggest it may be a waste product. They justify this statement by the fact that while the ink has an unpleasant “odious” smell, aplysioviolin itself is odourless. They suggest that a possible source of the odour, and also a candidate for the defensive agent, is a brominated aromatic compound, aplysinol.
NOTE an earlier author, cited here, also working on ink composition in A. californica, uses the term aplysioviolin for the main blueish-coloured component of the ink, but has an additional term aplysiorhodin for a reddish component, and adds a third term aplysioazurin for another blue componen (Winkler 1959). What the present status of all these components is at present will require a biochemist's determination
Chapman & Fox 1969 J Exp Mar Biol Ecol 4: 71
Winkler 1959 Pac Sci 13: 357
Research Study 3

Fig. 1. Threshold stimulus level (mild electrical shock) must be reached before inking occurs in Aplysia californica
Ink-release in Aplysia californica is a high-threshold, all-or-none, behaviour controlled by just a few neurons located in the abdominal ganglion. Note in Fig. 1 that a mild electrical shock must reach a threshold level before an animal inks. The ink is released into the mantle cavity and actively ejected from between the parapodia and the siphon by pumping movements of the mantle and parapodia (Fig. 2 photo series). The authors note that a healthy Aplysia never releases ink spontaneously.
NOTE but see comment #5 in Research Study 1 above

Fig. 2. Photo Series: an electric shock is applied just posterior to the head of an Aplysia californica, causing the sea hare to turn in the opposite way

The sea hare pulls in its head & expels ink from between its parapodia & from siphon

The ink is discharged in a series of pumping movements of the mantle and parapodia
Carew & Kandel 1976 J Neurophysiol 40: 692
Carew & Kandel 1973 Fed Proc 32: 368
Research Study 4

Fig. 1. Direction of ink release in Aplysia californica varies, depending upon where the aversive stimulus is applied
Ink release by sea hares Aplysia californica following stimulation is usually in the direction of the source of the stimulus. A white opaline secretion may often be released in company with the ink. Ink originates from the ink gland located on the mantle shelf just above the gills, while opaline secretion comes from glands located on the floor of the mantle cavity (Fig. 1). The secretions are directed to the front or back by contractions of the siphon, mantle, and parapodia as shown. Note the difference in the siphon orientation in the two scenarios. In a "tail-shocked" individual (Fig. 2) the siphon stays level and ink is pumped posteriorad by mantle contractions. Note that the ink is released posteriorly as the individual crawls away. In a "head-shocked" individual (Fig. 3) the siphon constricts and the mantle contractions in this case pump the ink secretions anteriorad through the flared margins of the parapodia. Here, an individual pulls in its head and ejects its ink towards the front. Durations of the series are 15 and 25sec, respectively, after application of the shocks.
NOTE includes pinching with forceps, electrical shock, and application of crystals of NaCl

Fig. 2. Photo series showing ink release in Aplysia californica after an aversive tail stimulus

Fig. 3. Photo series showing ink release in Aplysia californica after an aversive head stimulus
Walters & Erickson 1986 J Comp Physiol A 159: 339
Research Study 5
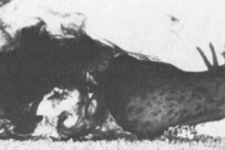
In laboratory confrontations the predatory Navanax inermis will readily attack and eat juvenile sea hares Aplysia californica. The Fig. 1 sequence photographed by the authors shows that shortly after contact by the predator, the prey is ingested by suction and swallowed whole. The entire sequence lasts less than 30sec. No ink is released by the sea hare during the attack, which may be relevant to the question posed in Research Study 6 below about the role of ink in defense.
NOTE Navanax lives much deeper than the shallow-dwelling A. californica, so it is not clear how much natural predation there would be; in fact, the sea hare shown here may not even recognise the Navanax as a predator

Fig. 1 Photo Sequence: on contact, the predator
Navanax inermis turns to face the prey sea hare
Aplysia californica

The sea hare is sucked in, with just its tail sticking out

Within 30sec the sea hare is ingested
Leonard & Lukowiak 1986 Behaviour 98: 320
Research Study 6

Fig. 2. A squirt of ink from a syringe onto a sea anemone Anthopleura sola stops it from consuming a sea hare Aplysia californica; a similar squirt of seawater has little or no effect on the sea anemone
Does the ink function in defense against predators? Research on species of Aplysia other than A. californica suggests that the ink is, at the very least, an irritant to other animals, but results are mixed from experiments to test whether it is actually deters feeding by potential predators. Experiments with A. californica and a potential sea-anemone predator Anthopleura sp. show that contact of a sea hare with the tentacles elicits copious ink discharge by the sea hare. Because of its component of sticky mucus, the ink tends to coat the sea hare and continues to be released even as the sea hare is ingested by the anemone (Fig. 1). In other experiments the ink triggers gastrovascular eversions in the anemones and causes them to reject whitefish, used to feed the anemones in the laboratory. If inkless sea hares are fed to sea anemones, many are eaten. However, if at the same time freshly collected ink from other sea hares is squirted onto the anemone, a significant proportion of the prey is rejected (Fig. 2). Sea hares without ink, but with normal skin/digestive-gland “chemistry”, are eaten more than sea hares with ink but no skin “chemistry”, suggesting that the ink is the more important defense. The authors note that sea hares tend to avoid ink, thus possibly removing themselves from areas of ongoing predation.
NOTE these experiments are done at the University of Miami using sea hares raised from eggs at the University’s Aplysia Mariculture Facility and anemones shipped in from California. The anemones are described as being A. xanthogrammica, but one of the researchers acknowledges in a later paper that the identification was likely mistaken, and that the anemones used were probably Anthopleura sola (see Research Study 9 below)
NOTE inkless specimens are obtained in two ways, the first by feeding sea hares green alga Ulva that lack the necessary phycoerythrobilin pigments used to manufacture ink. These inkless animals also lack any skin "chemistry" or digestive-gland “chemistry”. The second way is to “de-ink” is by massaging the ink gland over successive days. This eventually discharges all ink but leaves the sea hare with its complement of potentially toxic skin and digestive-gland secondary metabolites obtained from its normal diet of red algae

Fig. 1. Phoro series begins with a sea hare approaching a sea anemone in an aquarium tank...

...the Aplysia is caught up in the sea-anemone's tentacles...

...and ingested. The sea hare releases copious amounts of ink, noted within the manubrial region around the sea anemone's mouth...

...that shortly is seen diffusing from the anemone's gastrovascular cavity via the mouth..

...the sea hare crawls out, seeming not much the worse for wear.
Nolen et al. 1995 J Comp Phys A 176: 239
Research Study 7

Fig. 1. Components of the ink gland in Aplysia californica: RPV: Red-Purple-Vesicles; CV: Clear Vesicles; AV: Amber Vesicles
A detailed study of the processing, storage, and secretion of ink by Aplysia californica by scientists in Florida leads to the following: the purple-pigment component of the ink is a phycoerythrobilin, and is stored, along with a protein of unknown function in muscular ink-release vesicles within the ink gland. The ink gland is located in the mantle-shelf part of the mantle cavity and, when the vesicles discharge, the ink is carried out in the exhalent respiratory flow through the siphon and parapodial margins. After a red alga such as Gracilaria tikvahiae is consumed the ink pigment phycoerythrobilin is cleaved from its protein component in vacuoles in cells of the digestive gland and carried in the hemolymph to the ink gland. The ink is transported to and stored in membrane-bound vacuoles in the ink gland for variable lengths of time, then later incorporated into ink-release vesicles. The protein component of the ink, representing 35% of the dry mass of the secreted ink, is added at this time. Variable amounts of mucus are also released with the ink component. The diagram in Fig. 1 shows the different kinds of vesicles present in the ink gland, the main ones being the so-called Red-Purple Vesicles (RPV) that contain the ink. Also present in the ink gland are Amber Vesicles and Clear Vesicles. The ducts and pores from the Red-Purple Vesicles are shown, along with valves that control release of ink from them (refer to Coelho et al., 1998 for details of metabolism of phycoerythrins in the digestive gland of Aplysia californica).
NOTE the sea hares used in the study come from a culture facility at the University of Miami, NIH National Resource for Aplysia, Miami, Florida
NOTE in a later paper the senior author at the University of Miami provides a similarly detailed description of the opaline gland in A. californica, an organ whose function is much less well known than the ink gland (Prince, 2007)
Prince et al. 1998 J Exp Biol 201: 1595
Coelho et al. 1998 J Exp Biol 201: 425
Prince 2007 J. Moll Stud 73: 199
Research Study 8

Fig. 2. Aplysia californica inking

Fig. 3. Efficacy of escape of sea hares Aplysia californica from spiny lobsters Panulirus interruptus when one or the other, both types, or neither type, of secretion is released

Fig. 1. Aplysia californica being attacked by a spiny lobster Panulirus interruptus (from the authors' video)
Sea hares Aplysia californica appear to defend themselves from attack by spiny lobsters Panulirus interruptus by releasing ink and opaline secretions (Fig. 1). The authors use a novel experimental approach. They remove ink and/or opaline glands from test individuals and present the treated1 sea hares to the lobsters. Results show that sea hares with both glands intact, or only opaline gland intact, escape predation in about 64% of the encounters with spiny lobsters, while sea hares with neither gland or with just an ink gland escape predation in only about 18% of encounters (seeTable of data, representing only part of a much larger data set). Encounters in which sea hares release secretions and survive indicate that the secretions have different effects on the lobsters, with ink inducing ingestive behaviour, opaline secretion inhibiting ingestive behaviour and also evoking escape responses, and both secretions stimulating grooming by the lobster. Fig. 2 shows how an attack by a lobster on a sea hare would llikely end. After much release of ink the sea hare is allowed to escape and the lobster displays feeding behaviour. The active ingredients in the ink are millimolar quantities of amino acids that stimulate chemoreceptor neurons in the lobster’s nervous system. Included are large amounts of taurine, which is a known phagostimulant for many marine invertebrates, as well as lysine, and histidine. The secretions appear to function in at least three ways: the first, by a novel and previously undescribed form of chemical defense termed phagomimicry2, in which stimulation of feeding pathways deceive the lobsters into responding as though food stimuli were present (the behaviours include moving the first two pairs of legs to the mouth and “digging” movements); the second, by sensory disruption; and the third, by chemical deterrence. The authors describe phagomimicry as a “sensory trap” because the lobster’s chemosensory system is “trapped” to respond in a certain “preprogrammed” way. Although lobsters3 are not known to be major predators of sea hares worldwide, the study shows that the potential effects of ink-opaline secretions4 are far more complex than previously envisaged. What is needed now, of course, is comparable investigations of other potential predators of Aplysia californica (see Derby & Aggio, 2011 for an up-to-date review of chemical defenses in marine molluscs and other animals).
NOTE1 individuals with one of the secretory glands missing are still able to release secretion from the other and, according to the authors, all surgically treated animals appear to be “in good health” on the day following their surgery when they are used in experiments
NOTE2 videos of ink and opaline gland release, and induction of phagomimicry responses in the lobsters can be found at SEA HARES USE NOVEL ANTIPREDATORY CHEMICAL DEFENSES. The videos are found at the end of the article and are well worth watching, especially if you have never seen ink and opaline secretion being released by a sea hare
NOTE3 a study by researchers at Chapman University, California shows that an attack by lobsters Panulirus interruptus will act as a sensitising stimulus to the head/siphon withdrawal response in A. californica equal to that of a strong electric shock. Thus, in demonstrating that electrical shock does indeed mimic at least one natural stimulus, the authors provide an ecological context for its use in learning experiments with A. californica (Watkins et al., 2010)
NOTE4 ink and opaline secretions are acidic, ranging from pH 5 - 8. Other studies suggest that secretions of greater acidity lead to greater enhancement of the phagomimetic chemical defense (Shabani et al., 2007)
Kicklighter et al. 2005 Current Biol 15: 549
Derby & Aggio 2011 Integr Comp Biol 51: 771
Shabani et al. 2007 J Comp Physiol A 193: 1195
Watkins et al. 2010 J Neurosci 30 (33): 11028
Research Study 9
Researchers at Georgia State University reiterate the chemical makeup of ink and opaline secretions in Aplysia californica, and provide further detail on how the chemicals are packaged. The main defensive chemical is an L-amino acid-oxidase called escapin1. This compound is exclusively produced in the ink gland, has antimicrobial activity, and is thought by the authors to function chiefly in an antipredator role. Within the ink gland, escapin is only present in the so-called amber vesicles and not in the red-purple vesicles2 (refer to figure in Research Study 7 above). These latter contain the phycoerythrobilin pigments derived from Aplysia’s red-algal food that give the ink its characteristic purple colour. Both of the chief amino-acid components of the ink, namely, lysine and arginine, are involved in escapin’s bacteriostatic effects, but only lysine is involved in its bacteriocidal effects. Whether these antibacterial properties play a significant role in the everyday biology of Aplysia, or whether they are just a side-effect of the ink's antipredatory chemistry is not known. Lysine is also present in opaline secretion but in much higher concentration than in the ink. On the strength of this, the authors propose that the lysine in the opaline secretion acts as a substrate for the enzyme escapin in the ink, and that the simultaneous3 release of ink and opaline allows for the generation of antipredatory defensive compounds “from innocuous precursors at the precise time they are needed”.
NOTE1 the major amino-acid components in escapin are L-lysine and L-arginine
NOTE2 a third type of vesicle, clear, is also present, but appears to be more common in sea hares eating green algae or other laboratory foods such as lettuce that lack the pigments necessary to produce purple ink
NOTE3 the authors’ assertion that ink and opaline are commonly released simultaneously by Aplysia may provoke comment from other researchers. Although this may be usual in A. californica, in other Aplysia species (e.g., Aplysia punctata, A. dactylomela, A. parvula, A. braziliana, to name a few) the two secretions seem often to be released as separate events, ink more readily than opaline. In fact, it is often difficult to stimulate a sea hare to release opaline as it appears to have a higher stimulus threshold. In one of the videos accessible in the paper cited in Research Study 8 above, however, A. californica can be clearly seen releasing both secretions simultaneously
Johnson et al. 2006 J Exper Biol 209: 78
Research Study 10

Fig. 1. Sea anemones Anthopleura sola in an aquarium tank (thus explaining their bleached appearance). This new species represents the old "solitary" form of the aggregating anemone Anthoopleura elegantissima. It is much larger than the aggregating form, has different colour patterns to manubrium and tentacles and is, of course, solitary

Fig. 2. Aversive responses of anemones Anthopleura sola to ink of sea hares Aplysia californica involve tentacle retraction and/or shrivelling. Note that opaline secretion has no observable effect
A follow-up to Research Study 6 above investigates which components of the ink and opaline secretions of Aplysia californica are aversive to sea anemones Anthopleura sola (Fig. 1), as demonstrated by tentacle shriveling and/or retraction. These behaviours would perhaps lead to anemones dropping ensnared sea hares. Sea hares are first fed the red alga Gracilaria ferox and then their ink and opaline-gland secretions are applied to sea-anemone tentacles in 50µl doses with a syringe, with the same volume of seawater being used as a control. Results show that while the ink is aversive to the sea anemones, the opaline secretion actually initiates feeding by the sea anemones (Fig. 2). Tests with algal extracts elicit no response in the anemones, suggesting that the aversive components are actually produced by the sea hares. One of the ink’s components, a protein called escapin, is tested alone and together with its substrate L-lysine, but neither elicits tentacle shriveling and/or retraction different from seawater controls. The authors conclude that escapin plays little or no role in defense, at least not against A. sola. By use of fractionation techniques the authors determine that several components, including both lipophilic and hydrophilic ones, may be involved in the aversive responses. The authors suggest that the multiple components in the ink of Aplysia spp. may explain why different potential predators are affected in different ways. For example, opaline secretion is phagostimulatory to sea anemones, but not to lobsters, and so on.
NOTE this is a major protein in the ink and has antimicrobial activity
NOTE also, in Research Study 8 above, components of the ink of A. californica are found to act as phagomimics, that is, the lobsters exhibit feeding behaviours in the presence of the ink, but not directed to the sea hares. Other research on a related species Aplysia dactylomela in Jamaica finds that sea-hare inks act as sensory irritants to various invertebrates and fishes (Carefoot & Pennings, 1999)
Kicklighter & Derby 2006 J Exp Mar Biol Ecol 334: 256
Carefoot & Pennings 1999 J Exp Mar Biol Ecol 234: 185
Research Study 11

Fig. 1. Catfish Ariopsis felis

Fig. 2. Bluehead wrasse Thalassoma bifasciatum
Related studies by researchers at Georgia State University on defensive functions of ink and opaline secretions of sea hares Aplysia californica provide evidence that the aplysioviolin and phycoerythrin components are deterrents to feeding by different fishes. By recording impulses from nerves that innervate the chemosensory barbels1 of the marine catfish Ariopsis felis (Fig. 1) the authors determine that both ink and opaline components strongly reduce responses of the barbels to amino acids and bile salts, both known to be stimulatory compounds for teleost taste systems. Complementary studies on the tropical bluehead wrasse Thalassoma bifasciatum (Fig. 2) show that release of ink both interferes with the fish’s olfactory sensitivity and, when incorporated into food pellets2, with taste sensation within the mouth. When the wrasse’s olfaction is occluded by obstruction of the nares with petroleum jelly, the deterrent effect of the ink cloud is eliminated but not the ink’s effect on food acceptance. Thus, ink but not opaline secretion interferes with the olfactory-capture phase of feeding but not with the nonolfactory-acceptance phase. These studies3 add in some ways to our understanding of the functions of ink and opaline secretions in sea hares.
NOTE1 catfish have taste buds not only on the barbels, but also on the face, mouth, and general body surface
NOTE2 the ink components are incorporated into pellets made from shrimp powder and agar
NOTE3 the researchers’ choice of fishes to test is unusual, as neither species lives even in the same ocean as sea hares A. californica, let alone (in the case of bluehead wrasses) utilises sea hares of any species as food. These wrasses are primarily planktotrophic, subsisting mainly on copepods and various larvae, and sometimes small benthic crustaceans and snails. Also, a more logical choice for ink and opaline secretions to use on wrasses would be Aplysia dactylomela and, for catfish, A. brasiliana, because both pairs would then be cohabitants of the same oceans with potential for at least some history of co-evolution. The authors presumably choose California sea hares for the vast body of knowledge available on their ink and opaline secretions but, in the end, what have we learned that is useful?
Nusnbaum & Derby 2010 Anim Behav 79: 1067
Nusnbaum et al. 2012 J Comp Physiol A 198: 283






























 Nudibranchs & relatives
Nudibranchs & relatives