There are two main types of asexual reproduction in sea anemones, longitudinal fission and pedal laceration.
Research Study 1: Longitudinal fission

A clue to the asexual origin of aggregations of Anthopleura elegantissima was provided by early observations that the members of a given aggregation have identical colour pattern and sex (Fig. 1).
Francis 1973 Biol Bull 144: 64.
Research Study 2: Longitudinal fission


Aggregations of the intertidal sea-anemone Anthopleura elegantissima are formed through asexual division (longitudinal fission) of individuals. To divide, the two halves of an individual appear simply to crawl away from one another and, in time, to split in two (Fig. 1). The offspring are genetically identical clones, and these often exist on the shore in large aggregations separated from one another by distinct interclonal boundaries. Fission takes between 1 - 8wk, and occurs generally once per year during autumn and winter when food is scarce. Clonal aggregations may take years to become established, but can persist for several decades.
Sebens 1983 Sebens Pac Sci 37: 121
Ferrell 2005 Oecologia 142: 184.
Research Study 3: Longitudinal fission

The fission leaves a scar of connective tissue on Anthopleura elegantissima that can be recognised for 2 - 6mo and thus provides an historical record of the occurrence of the process in a population (Fig 1). Additionally, frequency of division in a population is positively correlated with a seasonal decrease of basal diameter. In San Juan Island, Washington, for example, monitoring of these parameters shows that asexual division is least common during spring and early summer when the anemones are rapidly growing, and most common from late summer through the winter, when they are growing only slowly.
Research Study 4: Longitudinal fission


Although Anthopleura elegantissima is commonly seen in its aggregated or clonal form, another form also exists,
Sebens 1983 Ecol Monogr 53: 405
Smith & Potts 1987 Mar Biol 94: 537
Geller & Walton 2001 Evolution 55: 1781
McFadden et al. 1997 Mar Biol 128: 12.
Pearse & Francis 2000 Proc Biol Soc Wash 113: 596.
Research Study 5: Longitudinal fission
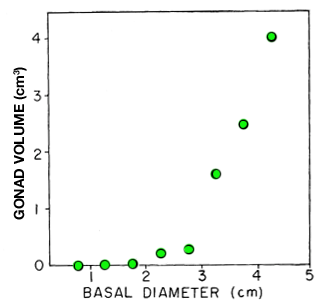
Further studies of asexual reproduction in Anthopleura elegantissima in San Juan Islands and Tatoosh Island, Washington show that rates are greatest during autumn and winter, averaging about 0.2 divisions per clonal individual per year at all study sites. Only the larger individuals in a clone divide. Clones in more favourable habitats produce larger individuals and these clones therefore have greater reproductive output. In comparison, clones in marginal habitats such as the high intertidal region are composed of small individuals, with the lowest rates of asexual division and lowest rates of reproductive output. Note in Fig. 1 that reproductive output is significantly higher in older, larger individuals of A. elegantissima. Based on rates of division and rates of disappearance from monitored clones, the author estimates
Research Study 6: Longitudinal fission
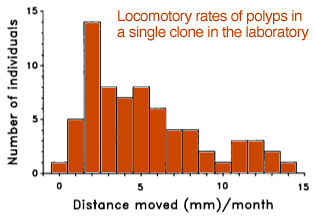

After the polyps of Anthopleura elegantissima or Corynactis californica (Fig. 1) divide, they move slowly away from the colony centre, thus providing room for further growth. Laboratory measurements of polyp movement in Corynactis californica at the Bodega Marine Laboratory, California indicate a mean rate of 5mm . mo-1 (Fig. 2), much slower than in actiniarian polyps such as Anthopleura. Corallimorpharians apparently lack the basilar muscles present in actiniarians, so perhaps some of the movement is by colony-wide “tidal” flow from increase in mass in the central part of the colony. The authors also report fission rates in the laboratory (at Santa Cruz, California) of once every 2mo, a rate that is intermediate between sea anemones and stony corals.
Test Your Understanding
- Reduces water loss in intertidal aggregations.
-
Sites the acrorhagal-rich warrior polyps on the periphery where they can better defend against attack from predatory snails.
NOTE all polyps of A. elegantissima possess special aggressive tentacles known as acrorhagi located around the upper column, just below the line of feeding tentacles. These are densely packed with large, potent nematocysts. The role of acrorhagi is considered elsewhere: AGGRESSION
- Allows for rapid/effective space utilisation.
- Enables cooperative prey capture.
- Maximises reproductive effort.
Research Study 7: Longitudinal fission


A publication by researchers at Shannon Point Marine Laboratory, Washington shows that the reproductive strategy of sea anemones Anthopleura elegantissima is influenced by the particular species that it hosts as a symbiont and the degree of
Research Study 1: Pedal laceration

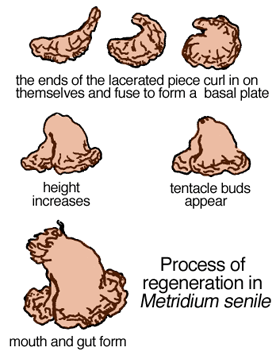
Some sea-anemone species, such as Metridium senile (Fig. 1) reproduce asexually by leaving bits of themselves around to grow into fully functional individuals (Figs. 2 - 4). The offspring are genetic clones of the adult. Sometimes, it seems as if the basal disc (pedal area) simply gets snagged on something or possibly a portion of it is released through contraction of pedal musculature.



Research Study 2: Pedal laceration

All forms of asexual reproduction in invertebrates rely on good powers of regeneration, a characteristic found in many primitive invertebrates. The Urticina lofotensis shown in Fig 1 ate a basket of snails floating above it in a shallow aquarium, intended to be used in a classroom experiment at the University of British Columbia. The only way to retrieve the snails was to cut the anemone open lengthwise with a scalpel. The snails were fine, as was the anemone after just a single day of healing. No attempt was made to stitch the wound, but the edges healed perfectly overnight with no visible scars. The specimen lived for several more years before being returned to the sea.
Research Study 3: Pedal laceration

How effective is a pedal-laceration type of asexual reproduction in plumose anemones Metridium senile? This is investigated by a researcher at Bodega Marine Laboratory, California for two populations, one intertidal; the other, growing on harbor floats. Results over 1yr of observation showed that each member of the intertidal population average about 15 pedal lacerations per month while the harbor-float population averaged about the same but with much larger variability, Thus, while the intertidal population varied in lacerations from 12 - 22 per individual per month, the harbor-float individuals averaged from 6 - 33 lacerations per month. With these remarkably high rates of asexual reproduction it is easy to see how large clones of genetically identical individuals can become so common (Fig. 1). Furthermore, while it had long been thought that pedal laceration in anemones was greater in habitats with more
Anthony & Svane 1995 Mar Ecol Progr Ser 124: 171
Research Study 4: Pedal laceration

Polyps of the corallimorpharian Corynactis californica also reproduce asexually by pedal laceration or budding, and canform extensive genetically identical clones (Fig. 1).
Research Study 5: Pedal laceration
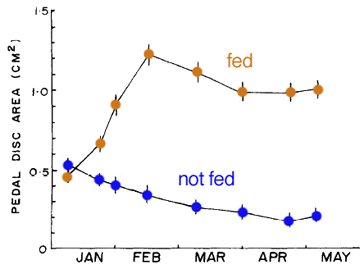
A detailed laboratory study on three species of Metridium by a researcher at Bodega Marine Laboratory, California provides comparative information on maximum size attained and asexual reproductive proficiency. Metridium exilis is a small intertidal species (typically 0.2cm2 pedal disc area) that reproduces asexually by binary fission (longitudinal fission). An ad libitum diet of adult brine shimps increases the rate of asexual reproduction, but has only a small effect on maximum size (1.2cm2; Fig. 1). The author notes that sexual reproduction in M. exilis may be quite
Research Study 6: Pedal laceration

In temperate latitudes the sea anemone Urticina crassicornis (Fig. 1) reproduces sexually by releasing gametes that fertilise externally, leading to a planula larva and to later settlement and metamorphosis. Recently, however, a group of Polish and Russian scientists believe they have identified aggregations of this species in intertidal zones of the Barents Sea that appear to originate asexually from a few large brooding females. The authors point to colour-pattern similarity among members of an aggregation, a size gradation from small to large at increasing distance from the purported large brooders, and observations of juvenile anemones both within the coelenteron (gastrovascular cavity) and appearing on the outer column of these same adults after emergence from their mouths (Figs. 2 - 3). The authors additionally point to the possibility that the monochromatic aggregations result from asexual reproduction, but how and where this might occur are not specified. These would be fascinating findings were it not for the absence of corroborative molecular or other evidence to support the assertion that the species in question is, indeed, U. crassicornis and not another species . This should be a good follow-up research project for the authors.


 Sea anemones & relatives
Sea anemones & relatives