
Fig. 1. Three-day planula larva of anemone Anthopleura elegantissima (100 x 150µm). The mouth of the larva is at the Left end
Sea anemones reproduce both sexually (considered for several genera of west-coast anemones in this section) and asexually, and some species do both. Sexes are mostly separate in sea anemones and their relatives. Gonads develop on the internal mesenteries and gametes are released via the mouth during summer or throughout the year, depending upon species and area. Development leads to a free-swimming larva called a planula (Fig. 1) that may feed or not feed depending upon species.
NOTE lit. “wandering” G.
Sexual reproduction in anemones is dealt with in alphabetical order of Genus below, along with Other anthozoans: Corallimorpharia & Alcyonacea
Research Study 1: Anthopleura

Fig. 1. Cluster of aggregating anemones Anthopleura elegantissima

Fig. 2. Gonad indices of sea anemones Anthopleura elegantissima in relation to seasonal seawater temperatures
In sea anemones the gonads grow on the internal mesenteries. A study on Anthopleura elegantissima (Fig. 1) in north San Francisco Bay, California shows that gonads build in size through spring and summer, and spawning occurs in late summer/early autumn. Gonad indices peak coincidentally with high surface seawater temperatures (Fig. 2). Eggs are released in brown mucousy masses, while spermatozoa are released in milky-white masses. Interestingly, in this early publication the author comments on finding aggregations of different sexes of A. elegantissima and makes reference to the possibility that they may be clones produced asexually through binary fission from a single individual. Further, the author notes that when individual anemones from different aggregations are mixed together in the laboratory, they eventually separate. This seems to be one of the earliest references describing the phenomenon of asexual cloning in A. elegantissima.
NOTE gonad index is calculated as the ratio of gonad volume to live mass of animal x 100
Ford 1964 Pac Sci 18: 138.
Research Study 2: Anthopleura

Fig. 1. Oocyte-diameter cycles in different seasons for sea anemones Anthopleura elegantissima in Bodega Bay, California
A similar reproductive pattern is exhibited by Anthopleura elegantissima in Bodega Bay, California. Here, monitoring of oocyte diameter in the gonads on the mesenteries reveals size increase during spring/summer, with spawning in late summer coincidental with peak seawater temperatures (Fig. 1). Spermatozoa remain viable in the gonads for up to 4mo. There is some evidence that males begin spawning earlier than females, and perhaps this stimulates the females to spawn. In 3yr of study the author notes finding only one individual with both male and female gonads.
NOTE a study on this species in Morrow Bay, California by the same author shows that levels of tissue lipid fluctuate in a manner roughly paralleling the reproductive cycle. Lipids are important energy-storage substances in many marine invertebrates, and their levels commonly cycle in synchrony with reproduction
Jennison 1979 Can J Zool 57: 403
Jennison 1979 J Exp Mar Biol Ecol 39: 211
Research Study 3: Anthopleura

Fig. 2. Graphs of gonad indices for sea anemones Anthopleura elegantissima at different tidal heights at Tatoosh and Lopez Islands, Washington. Data for A. xanthogrammica (not shown) are similar. "Low" and "high" refer to intertidal levels

Fig. 1. Great green anemone Anthopleura xanthogrammica in a tidepool
Studies in Washington show that gonads in two species Anthopleura xanthogrammica (Fig. 1) and A. elegantissima are produced through late winter, spring, and summer, with spawning in late summer and autumn. Fig. 2 shows that the pattern of percentage-volume change of gonads in A. elegantissima is similar whether on the open coast (Tatoosh Island) or inland (Lopez Island), and whether individuals are located high in the intertidal region, or low. Spawning is epidemic in a population and seems to be initiated by male sperm release. The gametes are released from the mouth, and drift away in the current (Figs. 3 - 4)
NOTE the gonads in sea anemones grow on both sides of internal mesentaries. The author fixes and sections sample gonads, then estimates percentage volume of each gonad as the volume of a cylinder

Fig. 3. Eggs being released from a female buried anemone Anthopleura artemisia in California
Courtesy Allison Gong, California

Fig. 4. Sperm being released from a male buried anemone Anthopleura artemisia in California
Courtesy Allison Gong, California
Sebens 1981 J Exp Mar Biol Ecol 54: 225.
Research Study 4: Anthopleura

Fig. 1. Anthopleura elegantissima in and around an open-coast mussel bed, photographed at high tide

Fig. 2. Anthopleura xanthogrammica in a surge channel with ochre stars Pisaster ochraceus
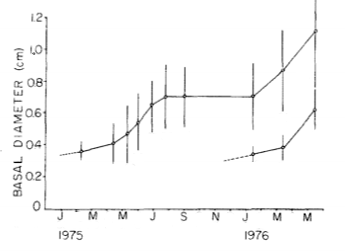
Fig. 3. Change in basal diameters of two cohorts of Anthopleura elegantissima in San Juan Islands, Washington
Further studies in Tatoosh Island and San Juan Islands, Washington suggest that both Anthopleura elegantissima (Fig. 1) and A. xanthogrammica (Fig. 2) preferentially settle in mussel beds, and that settlement may be quite irregular from year to year. One massive settlement of A. elegantissima at Tatoosh Island in late 1972/early 1973 could be followed for several years afterwards. During the several-year study on Tatoosh Island, numbers of A. elegantissima in the mussel beds more than doubled, mostly owing to a wholesale wave of asexual fission in the cohort in July 1975. During the 5yr study the author reports no settlement of A. xanthogrammica in the Tatoosh Island area, although juveniles are seen within the mussel beds. If areas are cleared near to and below the mussel beds in San Juan Islands, the juveniles crawl out into the clearings from the beds. Two distinct annual cohorts are visible in these data, each arising from winter settlement (November-March) and size measurements over time show strong seasonal differences in growth, with a similar pattern being present for both cohorts. Anthopleura xanthogrammica also recruit to mussel beds, then crawl downwards into tidepools and surge channels when they reach a larger size. The author suggests that the migration of juveniles of both species from the mussel beds is likely driven by the need for light for their photosynthesing symbionts.
Sebens 1982 J Exp Mar Biol Ecol 59: 103.
Research Study 5: Anthopleura

Fig. 1. Early planula of Anthopleura xanthogrammica feeding on a mixture of plastic microspheres and unicellular algae. Arrow shows food particles being caught on a trailing thread of mucus

Fig. 2. Planula of Anthopleura xanthogrammica ingesting particles
As described in a study in San Juan Islands, Washington, the planula larvae of both Anthopleura elegantissima and A. xanthogrammica feed on food items from the plankton, catching them on a trailing thread of mucus. The thread extends from the mouth and attaches to organic particles that are later drawn in with the mucus (Figs. 1 - 2). The planula is free-living for several weeks, then settles to the sea bottom and metamorphoses into a polyp.
NOTE "food" in the study food consists of plastic micro-spheres and unicellular algae. Although both types of particle are pulled into the mouth on the mucous line, the larvae subsequently reject them, so feeding is intimated but not actually demonstrated in the larvae of these species in the study
Siebert 1974 Can J Zool 52: 1383.
Research Study 6: Anthopleura

Fig. 1. Planula larva of a sea anemone Anthopleura elegantissima, but without an apical tuft of cilia. The anterior end is to the Left; the oral end to the Right
A description of the nervous/sensory system of the planula larva of Anthopleura elegantissima indicates a complexity comparable to that found in some adult sea anemones and hydroids. At the front end of the larva is an apical sensory tuft closely associated with an underlying apical sensory “organ” and an endodermal nerve plexus. Another set of ectodermal sensory cells are positioned at the oral end of the larva. The authors suggest that recognition of edible particles is likely to be by these ectodermal sensory cells. As the planula swims along the sea bottom near the end of its free-swimming life the apical tuft sways from side to side and is directed downwards suggesting a tactile sensory purpose possibly associated with settling.
NOTE this term is shown in quotation marks, for the reason that cnidarians do not have true organs. Simply put, organs consist of mesoderm, the third body layer in evolution, which is not yet present in these two-layered organisms
Chia & Koss 1979 J Morph 160: 275.
Research Study 7: Anthopleura

Fig. 1. Change in lipid content of tissues of sea anemones Anthopleura elegantissima in Morrow Bay, California over 2.5yr
A 2.5yr study on lipid levels in sea anemones Anthopleura elegantissima in Morrow Bay, California reveals fluctuations that roughly parallel the reproductive cycle. The same 6 clones (three male and three female) are sampled throughout the study, thus reducing genetic variability. The data show that lipid contents are independent of sex and directly proportional to size. Spawning times are in Aug - Sep; Fig. 1) and each is associated with a drop in percentage lipid, suggesting that lipid is involved in gamete production.
NOTE lipid contents are determined by mass loss after extraction of dried samples for 48h in a 5:1 mixture of ether/chloroform. Because it is not possible to separate the gonads from the other body parts, the method does not distinguish between gonadal and somatic lipids
Jennison 1979 J Exp Mar Biol Ecol 39: 211.
Research Study 1: Cribrinopsis

Fig. 1. Cribrinopsis fernaldi of indeterminate sex
Male Cribrinopsis fernaldi (Fig. 1) in San Juan Islands, Washington release sperm in springtime. The sperm swim or are drawn into the mouths of the females and fertilise the eggs, some of which are still in the gonads, while others are floating freely in the gastrovascular cavity, in the hollow tentacles, and in swellings around the upper outer surface of the body column. Development proceeds within the gastrovascular cavity through gastrulation (3d) to swimming planula larva (10d), and then to release of the swimming larvae via the mouth (15d). The authors note that the embryonic development of Cribrinopsis is similar to that of other anemones (e.g., Urticina crassicornis). The main difference is in the brooding behaviour of Cribrinopsis, which lasts for two or more weeks. The authors note the lecithotrophic nature of the eggs and suggest that the function of brooding is protective rather than nutritive.
NOTE at 20d in laboratory culture the planulae of Cribrinopsis fernaldi begin to settle in response to the presence of polychaetes Phyllochaetopterus sp., which are added to the culture vessels for this purpose. Why this particular substratum induces settlement is not known. It was used here because it worked to stimulate larval settlement in a another study by the junior author on a different species of anemone
Siebert & Spaulding 1976 Biol Bull 150: 128.
Research Study 1: Epiactis

Fig. 1. Epiactis prolifera with a few dozen offspring. Note that two of the juveniles have crawled off the adult. They will do this at the touch of a finger, but then generally crawl back

Fig. 2. Epiactis lisbethae with a few large juveniles still attached
Some west-coast anemone species brood their young, either outside on the body column, or inside on the mesenteries. The commonest of such species in northern Washington and British Columbia is Epiactis prolifera, which broods externally (Figs. 1 - 2). The species is unusual in that the offspring start out life as females, then become hermaphroditic. As an adult female, E. prolifera may be parthenogenetic, producing all-female offspring without the necessity of its eggs being fertilised or, rarely, be cross-fertile (presumably being fertilised by release of sperm from another hermaphrodite individual). Later, as an hermaphrodite, it is thought that an individual routinely fertilises itself. Self-fertilisation is not unheard of in the animal world, but it is probably something that should be looked into more carefully.
NOTE three other Epiactis species exist in British Columbia whose reproduction varies from external brooding with separate sexes (E. lisbethae), to internal brooding with separate sexes or with hermaphroditism (E. ritteri and E. fernaldi). Apparently, fewer than 20 brooding species of sea anemone exist out of about 800 species worldwide
NOTE lit. “virgin-producing birth”. Parthenogenesis always results in female offspring
Bucklin et al. 1984 Mar Biol 84: 175;
Edmands & Potts 1997 Mar Biol 127: 485.
Dunn 1975 Biol Bull 148: 199
Test Your Understanding
What advantage is it for Epiactis prolifera to be self-fertile?
[Click each option to see commentary]
-
Fertilisation is a certainty
Option a:
Yes. This can be especially advantageous in situations of low population densities and in harsh conditions such as the intertidal zone where cross-fertilisation may be difficult or impossible.
[hide]
-
Favours greater survival.
Option b:
Yes. Because the offspring carry the same genetic makeup as the parent, they are predisposed to live in the same habitat as the parent, which is obviously suitable for growth.
[hide]
-
Evolutionary success of the species is increased
Option c:
No, quite the opposite, as self-fertilisation prevents gene flow and may lead to expression of recessive genes. The development of diverse genotypes through cross-fertilisation and mutation lead to increased evolutionary potential to colonise new habitats.
[hide]
-
Allows colonisation of available patches in the habitat
Option d:
Yes. This goes along with the first two points. Widely dispersed individuals (i.e., out of cross-fertilisation “range”) can take advantage of free space that appears in the habitat (areas in otherwise crowded habitats may become free of attached organisms often by storms and log-battering or by actions of predators that eat an area free of prey).
[hide]
Research Study 2: Epiactis

Fig. 1. Yolky embryo of Epiactis prolifera caught up in an epithelial fold of the adult. The embryo would normally fit tightly in the fold but is shrunken because of fixation. It is about 300µm in diameter (one-third of a mm)

Fig. 2.
Epiactis prolifera bearing several large juveniles near the base of the column
Courtesy the author
In adult Epiactis prolifera the eggs and sperm mature simultaneously in gonads on the mesenteries and eggs are fertilised just prior to, or during, their release. Only a few dozen large eggs are produced and these are carried to the mouth, probably by cilia. On their release they tumble down the column of the anemone and, just above where the column joins the pedal disc, are caught up in folds of the epithelium (Fig. 1). If older juveniles are still on the column of the adult (Fig. 2), they may catch and eat the eggs, but later spit them out unharmed. The juveniles live and feed for about 3mo on the parent polyp before crawling off to take up solitary life.
Dunn 1975 Biol Bull 148: 199.
Research Study 3: Epiactis

Fig. 1. Epiactis sp. with several large juveniles, a few of which have moved off the parent onto the substratum surface
Often a gentle poke of the column of a sea anemone Epiactis prolifera with a finger can send the juveniles scattering onto the substratum (Fig. 1), but they later mostly return to the safety of the adult. It is not clear whether the youngsters return to the same parts of the adult they occupied previously, or to other parts. Studies at Bodega Marine Laboratory, California show that juveniles dislodged from the parent when smaller than 4mm basal diameter usually do not survive. The author notes that at any given time during the year in this area of California, 25 - 50% of adults are brooding.
NOTE colours may be quite variable in Epiactis prolifera. Studies in Bodega Bay, California indicate three colour morphs within a relatively small geographical area, with colour and size correlating with different types of substratum
Dunn 1977 Mar Biol 39: 41.
Dunn 1977 J Nat Hist 11: 457.
Test Your Understanding
The protective advantages to the brooding strategy of Epiactis are obvious, but what are the DISADVANTAGES?
[Click each option to see commentary]
-
There is less recruitment to the juvenile stage
Option a:
Yes. Reproduction by this method yields far fewer potential recruits than gamete-dissemination as found in other sea anemones but, on the flip-side, survival to the juvenile stage is virtually assured.
[hide]
-
There is limited genetic exchange
Option b:
No. The brooding has no connection with self-fertilisation (which is, of course, potentially genetically limiting)
[hide]
-
There is reduced potential for geographic colonisation.
Option c:
Yes. In the absence of a free-swimming larval stage, spread of the species to new habitats depends on the crawling ability of the juveniles. The advantage for brooders, of course, is that survival of the juveniles is enhanced because they are released into the identical habitat conditions that favour survival of the parent.
[hide]
Test Your Understanding
The main advantage of brooding in Epiactis prolifera seems to be in the safety provided by the adult’s protective umbrella of tentacles, and there is no known transfer of nutrients from the adult to the juveniles. This being the case, we might predict certain changes in a brooding adult’s morphology and/or behaviour that would favour survival of a brood, especially in areas where the risk of predatory attack is greater. Here are 8 possibilities, two of which are not so good. Think of which these not-so-good ones might be, then refer to the explanations provided for them.
[Click each option to see commentary]
Research Study 1: Metridium

Fig. 1. Aggregation of anemones Metriium senile, likely genetically identical clones
Studies on sexual reproduction in Metridium senile in Bodega Harbor, California shows that gonadal growth in females occurs from autumn to summer and peaks in August of each year. Spawning in both sexes occurs in Sept-Oct. Interestingly, while the pattern of gonadal maturation is asynchronous among three populations studied (one on a harbor float and two intertidal), spawning is synchronous.
NOTE a seasonal cycle is not so clear in males
Bucklin et al. 1982 Can J Zool, Lond 60: 3241.
Research Study 1: Stomphia

Fig. 1. Anemone Stomphia didemon
Studies at Friday Harbor Laboratories, Washington indicate that the swimming anemone Stomphia didemon (Fg. 1) spawns in April - May. The eggs, released from the mouth, are about 800µm in diameter, orange in colour, and filled with yolk. The eggs float at the surface for the duration of development. A blastula forms at 3d of age, a swimming planula at 5 - 7d, and settlement occurs at 8d (at 8 - 12oC). The larva then swims to the sea bottom with the apical end down and attaches. A basal disc forms and attachment is complete at 10d. By 13d of age tentacles appear, and by 90d the juvenile anemone has reached 1mm in diameter across the oral disc. Tests on preferred substrata for settling, using sand, gravel, and shell gravel, give uncertain results, although settling is somewhat quicker in their presence than in control seawater.
NOTE formerly thought to be a new species of Actinostola, the author formerly classifies it as Stomphia didemon sp. nov. Both genera are in the Family Actinostolidae. The author explains his choice of "didemon" as stemming from Gr. "cowardly or fearful", describing the species' response when touched by tentacles of S. coccinea. This origin of species name is hard to substantiate, as it seems the Gr. deilos is more commonly used for "fearful" (timid, coward, shy)
Siebert & Spaulding 1973 Pac Sci 27: 363.
Research Study 1: Urticina

Fig. 1. Seasonal gonadal indices of male and female Urticina lofotensis
In subtidal areas in Carmel Bay, California gonads in the sea anemone Urticina lofotensis mature during autumn, and spawning of gametes begins in early winter and extends through the year. Fig. 1 shows how gonad index (GI) changes with season.
NOTE GI is calculated as the ratio of mass of gonad divided by total body mass including gonad multiplied by 100 to give %
Wedi & Dunn 1983 Biol Bull 165: 458.
Research Study 2: Urticina

Fig. 2. Drawings showing planula larva swimming over surface of green alga Ulva with its aboral end foremost. Shortly after settlement a mouth, or stomodaeum, breaks through at the oral (upper) end. The cross-section shows the juvenile at 6h post-settlement, with the mouth forming, and the inner, endodermal, cells still packed with yolk

Fig. 1. Sexes are separate in the sea anemone Urticina crassicornis, as in most anemone species
Studies on laboratory-reared planula larvae of Urticina crassicornis (Fig. 1) show that the larvae will swim for as long as 18d without food and then settle onto pieces of green alga Ulva, possibly in response to bacterial films on the algae. After swimming and crawling about on the alga for a few moments, the larva settles on its hind (aboral) end, becomes squat in shape, and begins to develop a mouth (Fig. 2).
Stricker 1985 J Morph 186: 237.
Research Study 3: Urticina
Studies on development and early growth of the sea anemone Urticina crassicornis in San Juan Island, Washington indicate the following stages (at 12°C): spawning occurs in April - June and involves strings of mucus containing gametes being extruded from the mouth. The eggs first aggregate, sink, separate, then float to the surface. The eggs are yellow-tan in colour and about 600µm in diameter. The sperm become suspended in the water column soon after their release. Figs. 1 - 4 show post-fertilisation leading to the juvenile
NOTE juveniles 14mo old are 15mm diameter with 45 - 50 tentacles. The authors note that size is no sure indication of age, as growth is dependent upon amount of food eaten.

Fig. 1. Day 1 cleavage to 16-cell stage in Urticina crassicornis

Fig. 2. Day 8 planula larva of Urticina crassicornis (530 x 750µm)

Fig. 3. Day 10 the planula larva of Urticina crassicornis explores, and temporarily sticks to, the substratum. By Day 11 the planula settles and metamorphoses. The juvenile is 0.6mm diameter and begins to develop a mouth (protruding on the Right) and tentacle buds.

Fig. 4. Day 17 the juvenile Urticina crassicornis has four tentacles. Day 21 juveniles have 8 tentacles and commence feeding.
Chia & Spaulding 1972 Biol Bull 142: 206.
Research Study 1: Other anthozoans: Corallimorpharia & Alcyonacea

Fig. 1. Corallimorpharians Corynactis californica
An 18mo study of the corallimorpharian Corynactis californica (Fig. 1) in the Hopkins Marine Refuge, Monterey, California shows that female clones develop eggs from Aug-Dec. Male clones develop synchronously with the females. Spawning is in late Nov-early Dec, coincidental with rising seawater temperatures and likely cued by them. Fertilisation is external and development is to a free-swimming planktonic planula larva (140µm in diameter). The larvae are not reared to metamorphosis in the laboratory. The authors comment that their study is the first to be published on sexual reproduction in C. californica.
NOTE the authors note that C. california is the only west-coast species of corallimorpharian
Holts & Beauchamp 1993 Mar Biol 116: 129.































 Sea anemones & relatives
Sea anemones & relatives